Close
Welcome to PD Labs
PD Labs specializes in health & wellness products. Choose personalized care with a compounding pharmacy you can trust.

At PD Labs, we believe in empowering you to take control of your health. We’re committed to providing you with the knowledge, resources, and personalized solutions you need to live a healthier, happier life.
We compound unique medications for a wide range of conditions, including mold, Lyme, CIRS, autism, pain management, and more.
Our carefully curated selection of pharmaceutical-grade supplements, some formulated in-house, ensures you receive the highest quality products.
Our team of highly qualified pharmacists, doctors of pharmacy, and certified compounding technicians is committed to providing you with the best possible care.

Years of Experience

















Clinical Grade Products

Explore our curated selection of high-quality supplements, including vitamins, minerals, herbs, and specialty formulations
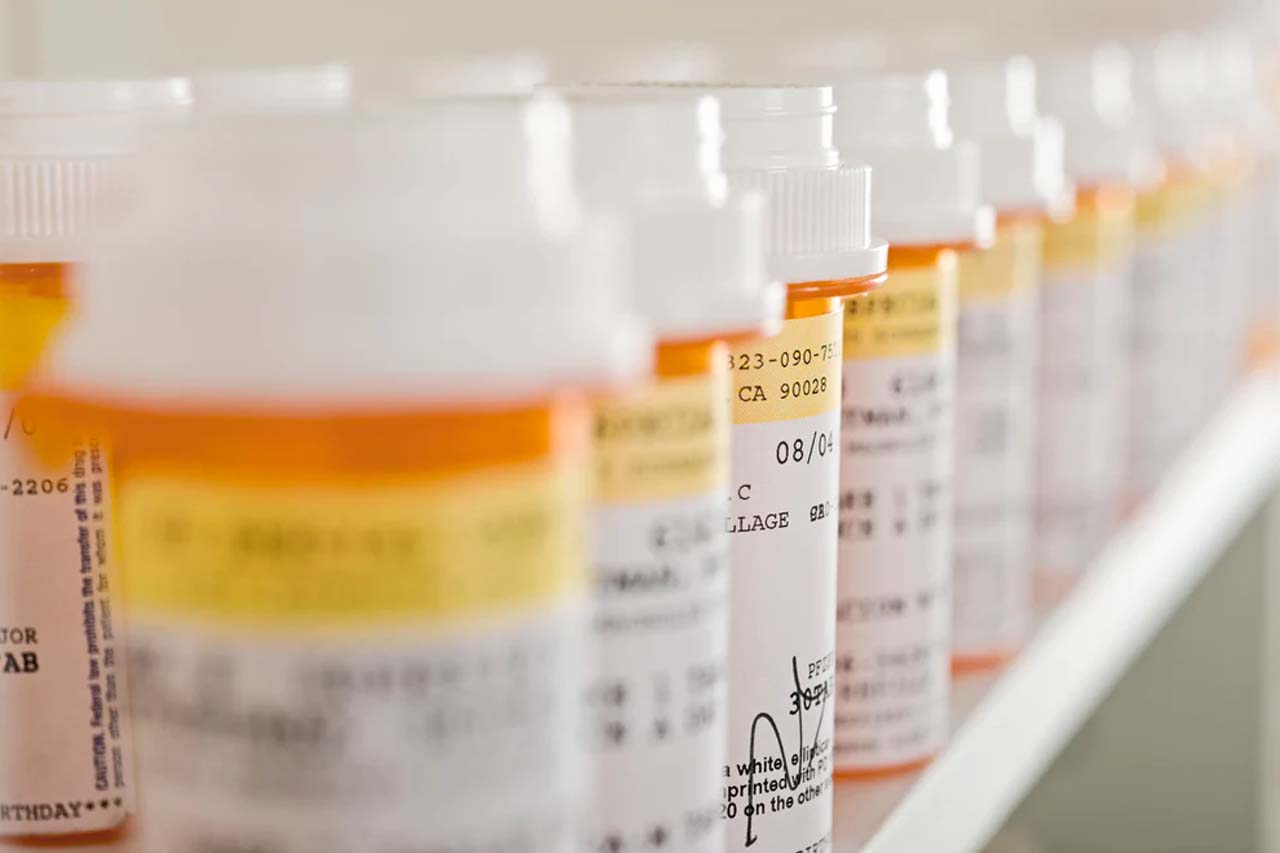
Need to refill your prescriptions? We offer convenient ways to renew.

We specialize in creating personalized medications tailored to your specific requirements.
Our experienced pharmacists and compounding technicians are dedicated to providing personalized care and support
Love PD Labs! Been getting compound prescriptions here for years. They are super friendly and responsive and professional. Their new location is excellent. They also now have a very wide selection of supplements to choose from. I sincerely highly recommend PD Labs!
it today’s world, excellent customer service is hard to come by. I’ve been working with PD Labs for 3 years now and every request or issue I’ve had has been handled promptly. they truly care about their customers. REFRESHING!!!
I have been using PD Labs for my prescriptions, compounding prescriptions and supplement needs for years. They are always pleasant to talk to and extremely knowledgeable. Being a business owner myself, I know mistakes are bound to happen…
Worked with another pharmacy who never got my thyroid meds correct. Ray took the time to listen to me and I’m happy to say I finally feel like myself again! Just wish we’d changed to PD Labs years ago!
Sign up for our newsletter to get up-to-date information, news & insights.